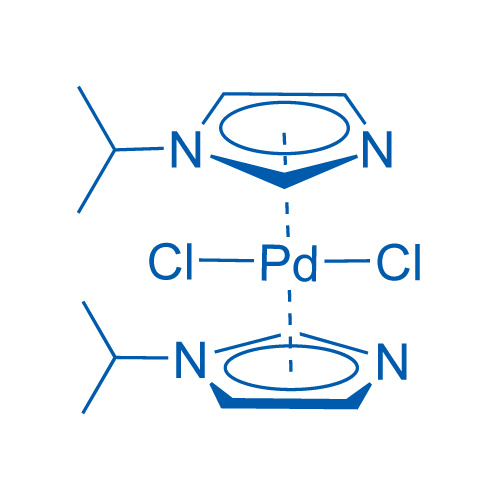
Dimetallic Palladium‐NHC Complexes: Synthesis, Characterization, and Catalytic Application for Direct C−H Arylation Reaction of Heteroaromatics with Aryl Chlorides - Lee - 2020 - Advanced Synthesis & Catalysis - Wiley Online Library
![Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML](https://www.mdpi.com/molecules/molecules-22-00399/article_deploy/html/images/molecules-22-00399-sch021.png)
Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML

The diimine ligand is coordinated to the palladium atom in a chelating,... | Download Scientific Diagram

Tetranuclear Palladium Complexes of Abnormal N‐Heterocyclic Carbene Ligands and their Catalytic Activities in Mizoroki‐Heck Coupling Reaction of Electron‐Rich Aryl Chlorides - Lee - 2019 - Advanced Synthesis & Catalysis - Wiley Online Library
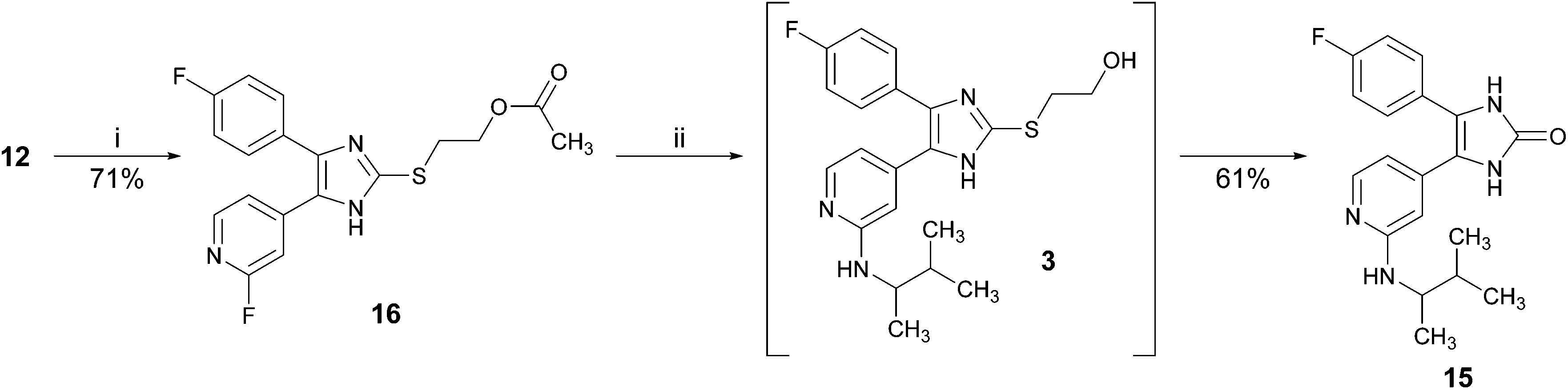
An optimized and versatile synthesis to pyridinylimidazole-type p38α mitogen activated protein kinase inhibitors - Organic & Biomolecular Chemistry (RSC Publishing) DOI:10.1039/C5OB01505G

Molecular structure of C2. Selected bond distances (Å): Pd(1)−C(11)... | Download Scientific Diagram

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Palladium‐Catalyzed C3 or C4 Direct Arylation of Heteroaromatic Compounds with Aryl Halides by CH Bond Activation - Roger - 2010 - ChemCatChem - Wiley Online Library

US20170240515A1 - A one pot process for synthesis of oxazoline and imidazole compounds from glycerol - Google Patents
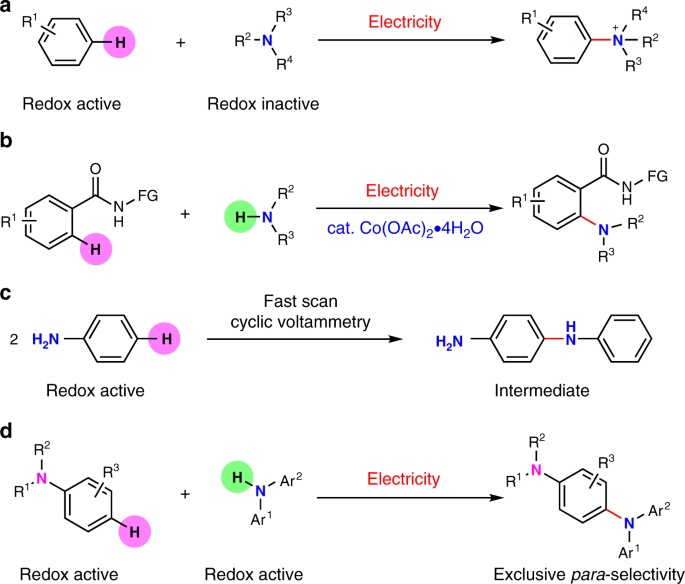
Electrooxidative para -selective C–H/N–H cross-coupling with hydrogen evolution to synthesize triarylamine derivatives | Nature Communications

Regiocontrolled Synthesis of 1,2‐Diaryl‐1H‐imidazoles by Palladium‐ and Copper‐Mediated Direct Coupling of 1‐Aryl‐1H‐imidazoles with Aryl Halides under Ligandless Conditions - Bellina - 2006 - European Journal of Organic Chemistry - Wiley Online Library

US20110028733A1 - Process for the preparation of 5-(2-ethyl-dihydro-1h-inden-2-yl)-1h-imidazole and salts thereof - Google Patents

Cycloheptyl substituted N-heterocyclic carbene PEPPSI-type palladium complexes with different N-coordinated ligands: Involvement in Suzuki-Miyaura reaction - ScienceDirect

Recent Progress in the Use of Pd-Catalyzed C-C Cross-Coupling Reactions in the Synthesis of Pharmaceutical Compounds

Are Imidazoles Versatile or Promiscuous in Reactions with Organophosphates? Insights from the Case of Parathion
![Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML](https://www.mdpi.com/molecules/molecules-22-00399/article_deploy/html/images/molecules-22-00399-sch013.png)
Molecules | Free Full-Text | Pharmacological Potential and Synthetic Approaches of Imidazo[4,5-b]pyridine and Imidazo[4,5-c]pyridine Derivatives | HTML
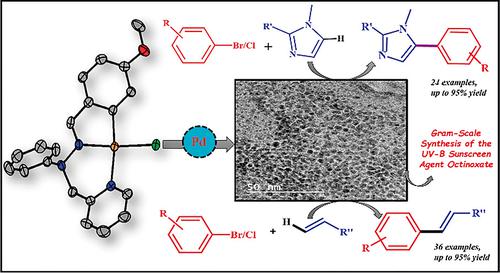
Palladium‐Based Catalysts Supported by Unsymmetrical XYC–1 Type Pincer Ligands: C5 Arylation of Imidazoles and Synthesis of Octinoxate Utilizing the Mizoroki–Heck Reaction - Eur. J. Inorg. Chem. - X-MOL
![Unexpected photochemical transformation of imidazole derivatives containing the 5-hydroxy-2-methyl-4H-pyran-4-one moiety. Environmentally friendly method for the synthesis of substituted imidazo[1,5-a]pyridine-5,8-diones - Tetrahedron Lett. - X-MOL Unexpected photochemical transformation of imidazole derivatives containing the 5-hydroxy-2-methyl-4H-pyran-4-one moiety. Environmentally friendly method for the synthesis of substituted imidazo[1,5-a]pyridine-5,8-diones - Tetrahedron Lett. - X-MOL](https://xpic.x-mol.com/20190826%2F10.1016_j.tetlet.2019.151080.jpg)
Unexpected photochemical transformation of imidazole derivatives containing the 5-hydroxy-2-methyl-4H-pyran-4-one moiety. Environmentally friendly method for the synthesis of substituted imidazo[1,5-a]pyridine-5,8-diones - Tetrahedron Lett. - X-MOL
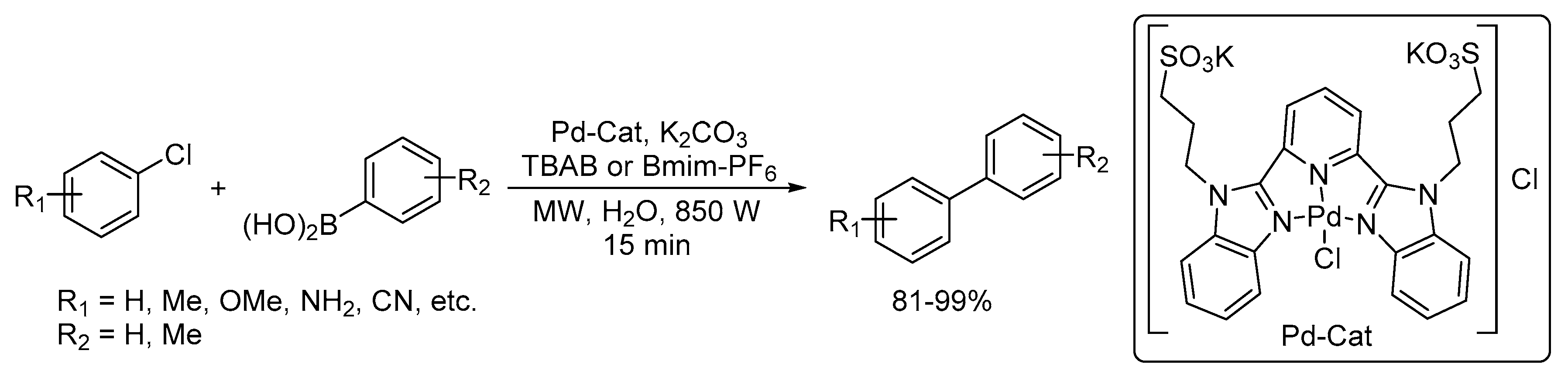
Catalysts | Free Full-Text | Microwave-Assisted Palladium-Catalyzed Cross-Coupling Reactions: Generation of Carbon–Carbon Bond | HTML

![Bis(1 methyl 1H imidazole κN3)[N,N′ o phenylenebis(pyridine 2 carboxamido) κ4N]manganese(II) Bis(1 methyl 1H imidazole κN3)[N,N′ o phenylenebis(pyridine 2 carboxamido) κ4N]manganese(II)](https://data01.123dok.com/thumb/methyl-imidazole-phenyl-enebis-pyridine-carbox-amido-manganese-z3dx218y.Pki4ZiPT5VIqTD1so.jpeg)